Abstract
Background: The incidence of chronic lymphocytic leukemia (CLL) in Sweden is about 500/year. Previous US data suggest that region and type of institution/hospital where patients were treated may affect outcome. Swedish results may differ as almost all CLL patients are treated within public health care, most treatment decisions are taken at therapy conferences and we have a widespread usage of yearly updated national CLL guidelines.
This study investigates the outcome following 1st line CLL therapy in a well-defined population of consecutive patients in a region with complete follow-up: such data may be used as quality assurance for healthcare providers and serve as real-world control for clinical trials, selecting patients based on inclusion/exclusion criteria. The Swedish Cancer Registry and the Information Network for Cancer (INCA) gives us a unique opportunity to identify all patients diagnosed within a defined time period, thus providing a possibility to obtain data on various therapies and outcome on a nation-wide basis.
Methods: This is a retrospective study on all Swedish CLL patients who received 1st line therapy due to progressive, symptomatic CLL. The time period 2007-2013 was selected to obtain sufficient follow-up. In-depth analysis of all individual patient files was performed. Cross-checked, computerized data were subject to statistical analysis. Endpoints were clinical response (IWCLL criteria), PFS, OS and safety. Type of treating hospital (local/regional/university) and geographical region was recorded. Whether choice of 1st line therapy was compliant to the national CLL guidelines was also recorded. A multivariate cox proportional hazards model was used to explore risk factors on outcome.
Results: Between 2007 and 2013, all 1st line treated patients (n=1053) were identified. At 1st line treatment initiation median age was 71 years (range 31- 96) and 34% were females. Rai stages (0-I, II-IV) were 45 and 53%. Regarding performance status (PS) 96% were grade 0-2 and only 2% had grade 3-4. FISH was not introduced in routine healthcare until late in the period and was excluded in the first analysis. IGHV mutational status analysis was not compulsory. Most frequently used treatments were chlorambucil (CLB) (39%), FCR (27%) and FC (16%). Bendamustine (B) was not introduced until end of the study period thus only 6% (n=63) had received B or BR. Other regimens included for example alemtuzumab (5%), CHOP/CVP +/-R (3%) and rituximab (R) single (2%). Dosing intensity was similar across geographical regions and type of institutions.
The ORR was 64% (15% CR); with CLB 43% FCR 84%, FC 75% and B/BR 75%. ORR was significantly associated with type of treatment and age but not with sex, Rai stage (p=0.051) or bulky disease.
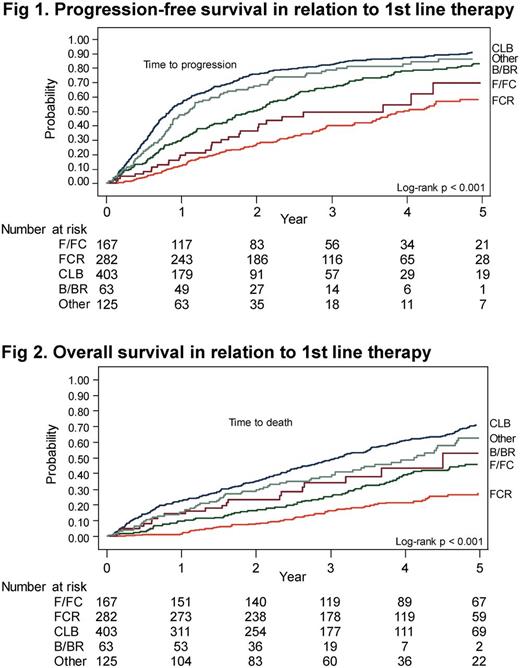
Median PFS was 19 months and at a median follow-up of 5 years, 49% of all patients remained alive. PFS and OS were both significantly associated with type of treatment (Fig 1 and 2); CLB-treated patients had a median PFS and OS of only 9 and 33 months. Usage of CLB declined during the study period, but still 1/3 of all patients (in some regions up to 44%) received 1st line CLB as late as 2013.
Age, Rai stage, PS and adherence to treatment guidelines were also significantly associated with PFS and OS by univariate analyses.There was no clearcut impact on PFS and OS by type of health care institution/hospital.
Multivariate analysis revealed that age, PS, treatment (all p=0.00001), Rai stage (p=0.003) and sex (p=0.02) were all significantly associated with OS, whereas adherence to guidelines was no longer significant.
Infections ≥ grade III were significantly associated with type of treatment (p=0.006); CLB 19% vs FCR 30%. Richter transformation occurred in 5% of the patients. 15 % of the patients were diagnosed with a secondary malignancy equally distrubuted between types of 1st line therapy.
Conclusion:
This is the largest retrospective cohort of consecutive 1st line treated CLL patients from a well defined geographical region (Sweden) with a complete follow-up. All patients were identified and data were subject to in-depth analysis of every individual patient file, ensuring high quality data. We demonstrate that single-agent CLB resulted in a poor outcome and conclude that alternative modern, effective 1st line treatment alternatives must be offered to elderly patients. This study also highlights the importance of updated evidence-based guidelines and their usage across all levels of health care.
Kimby: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Celgene: Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees. Andersson: Janssen-Cilag: Honoraria; Abbvie: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; roche: Speakers Bureau; GSK: Speakers Bureau. Karlsson: Abbvie: Honoraria; Janssen-Cilag: Honoraria. Mattsson: Janssen-Cilag: Honoraria; Gilead: Honoraria; Abbvie: Honoraria; Roche: Honoraria. Österborg: Pfizer: Honoraria; Abbvie: Honoraria; Celgene: Research Funding; Janssen-Cilag: Honoraria, Research Funding; Gilead: Honoraria, Research Funding. Hansson: Abbvie: Honoraria; Gilead: Honoraria, Research Funding; Janssen-Cilag: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.